Overview
The American Cancer Society Cancer Action Network (ACS CAN) empowers advocates across the country to make their voices heard and influence evidence-based public policy change, as well as legislative and regulatory solutions that will reduce the cancer burden. As part of this effort, ACS CAN deploys surveys to better understand cancer patient and survivor experiences and perspectives, through our Survivor Views research panel. The panel is a group of cancer patients and survivors who respond to regular surveys and provide important insights to support ACS CAN’s advocacy work at all levels of government.
Fielded September 12-25, 2023, our latest survey explores the impact of recent drug shortages on cancer patients and survivors, as well as experiences with telehealth and biomarker testing. The web-based survey was conducted among 1,222n cancer patients and survivors nationwide who have been diagnosed with or treated for cancer in the last seven years.
Key Findings
Drug Shortages
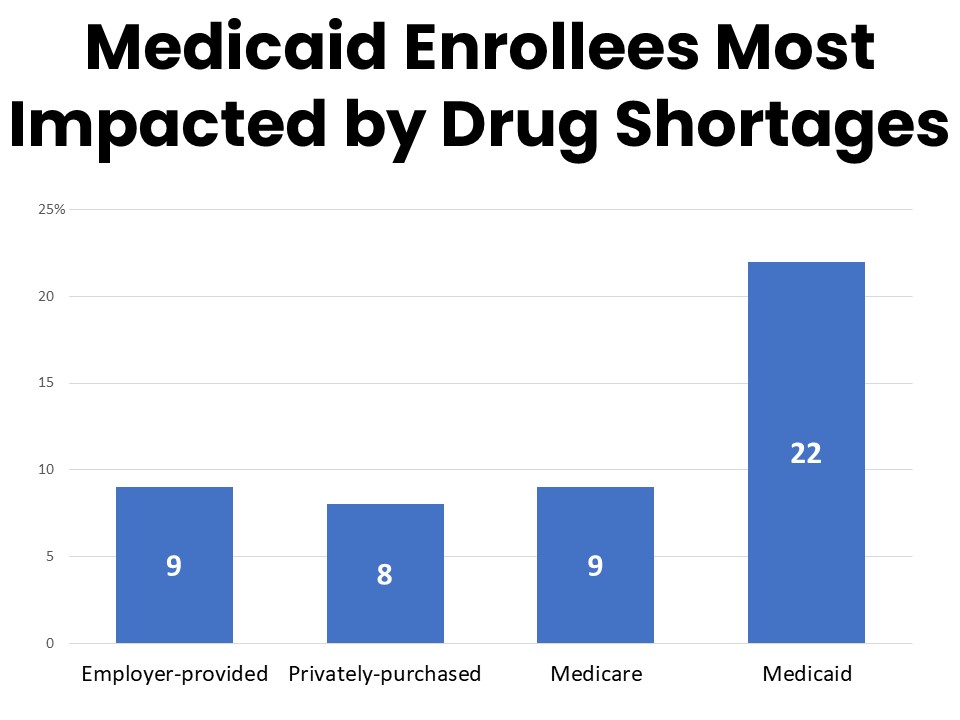
- One-in-ten of those in active treatment have been impacted by recent drug shortages, with Medicaid enrollees most likely to be impacted (22%).
- Those impacted cited difficulties finding substitute medications (68%), treatment delays (45%), and difficulties related to insurance coverage, such as coverage for an alternate therapy.
Biomarker Testing
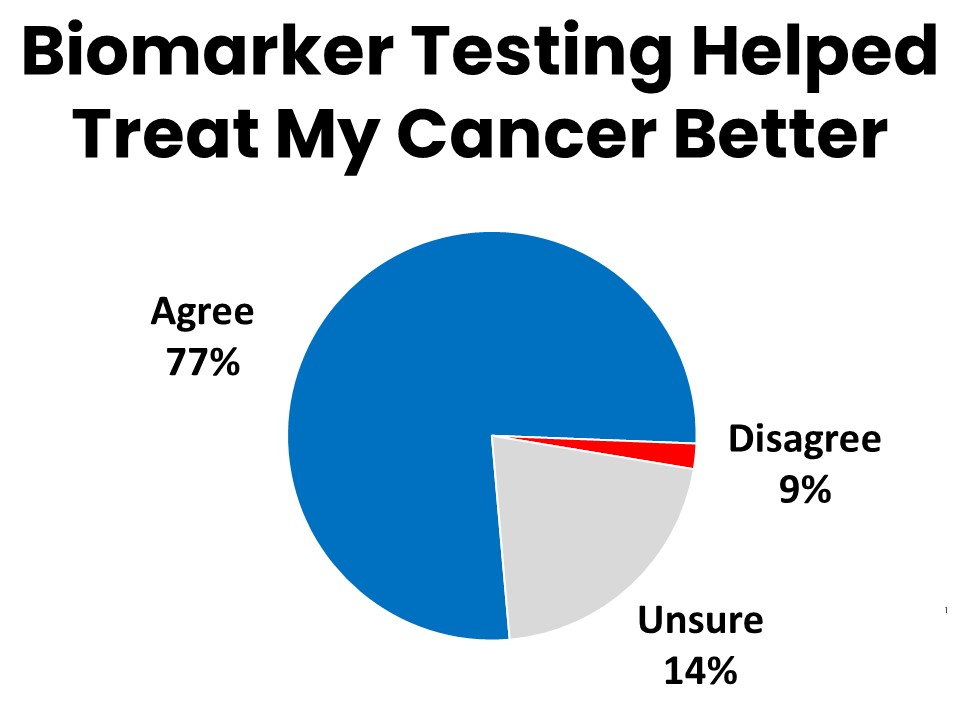
- Over three-quarters (77%) of survey respondents who have had biomarker testing agree that the results gave their provider valuable information that led to better treatment.
- 77% of those who have not had biomarker testing would like to have it if they were a good candidate for it, however most (69%) did not discuss it with their doctor.
- Nine percent did not have biomarker testing due to cost or coverage concerns.
Telehealth
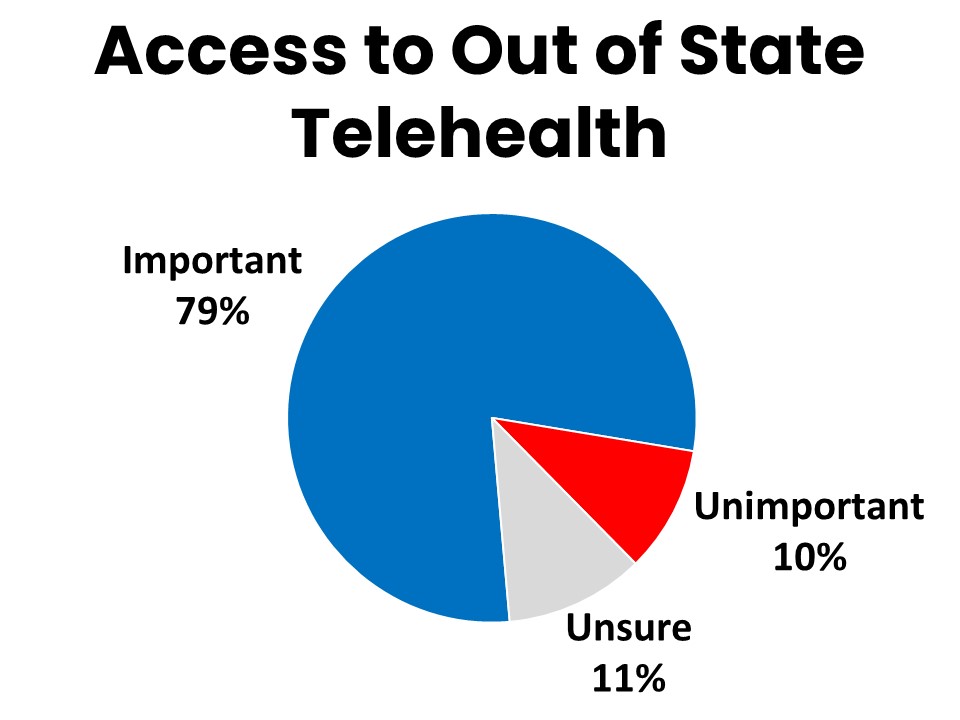
- Four-in-five agree it is important for cancer patients to have access to telehealth services from out of state providers.
Summary of Results
One-in-Ten Cancer Patients in Active Treatment Have Experienced Recent Drug Shortages; Medicaid Enrollees Impacted Most
Ten percent of survey respondents who have been in active treatment during the past twelve months have been impacted by drug shortages during that time. Those enrolled in Medicaid are significantly more likely to report being impacted by drug shortages at 22%. Among those who have been impacted by drug shortages in the past year, 57% say the shortage was related to their cancer care, including medications to treat cancer as well as its side effects and complications. Nearly a third of those experiencing drug shortages report the shortage was of a treatment medication such as chemotherapy or immunotherapy. Just over a quarter faced a shortage related to a psychiatric drug such as to treat anxiety, depression, or ADHD and 22% mentioned a shortage of a pain medication.
The most common impact among those facing a drug shortage related to their cancer was delayed or missed treatment, reported by 45%. Thirty-eight percent of those who faced delays in their cancer treatment report delays of a month or more. Twenty-three percent were able to use an alternate drug, while most (68%) were unable to identify an effective substitute. Thirty-five percent had difficulty using their insurance to fill a prescription related to the shortage, such as facing delays covering a substitute drug. Fourteen percent were ultimately able to find the drug before treatment was delayed, and six percent continued on the medication with a dose change.
Representative quotes on the impacts of the drug shortage:
“My doctor office only gets enough for one patient a month. I'm currently set to get mine in over six months.”
“It has extended my treatment which adds to the stress level and horrible side effects.”
“Had to have the shots implemented differently which caused a lot more discomfort and pain. The side effects were different and a bit worse for days following the treatment."
“When I can’t get my pain medication I can’t function properly throughout the day. My legs hurt and want to give out on me.”
Two-in-five of those facing drug shortages related to their cancer in the past year say the shortage has since resolved and their drug is now available. One quarter are continuing their treatment but with increased or unmanaged side effects due to the shortage. Twenty-two percent remain on a substitute drug or dosage and 2% report that their treatment is currently on hold while they try to find the drug or a substitute.
Patients and Survivors Overwhelmingly Agree Biomarker Testing Improved Their Treatment
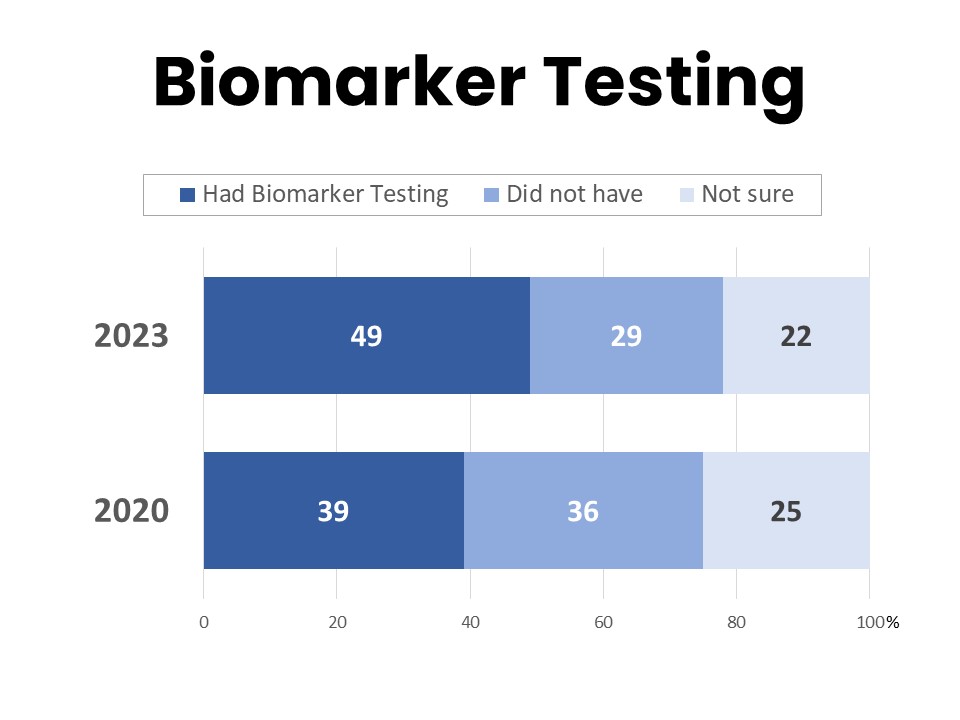
About half (49%) of those surveyed have had biomarker testing for their cancer, while 29% say they have not and 22% are unsure. This is an increase from 39% in 2020 who had biomarker testing.
Those most likely to have had biomarker testing include younger patients, those with higher incomes, and those with higher educational attainment. Among those who have had biomarker testing, 84% say their provider initiated the process and 14% report asking for the test. Those with college- or post-graduate degrees more frequently report initiating the conversation about biomarker testing with their provider, and patients and survivors with annual household income above $125,000 are twice as likely to report asking their provider about biomarker testing as those in the $50,000-$75,000 annual household income bracket.
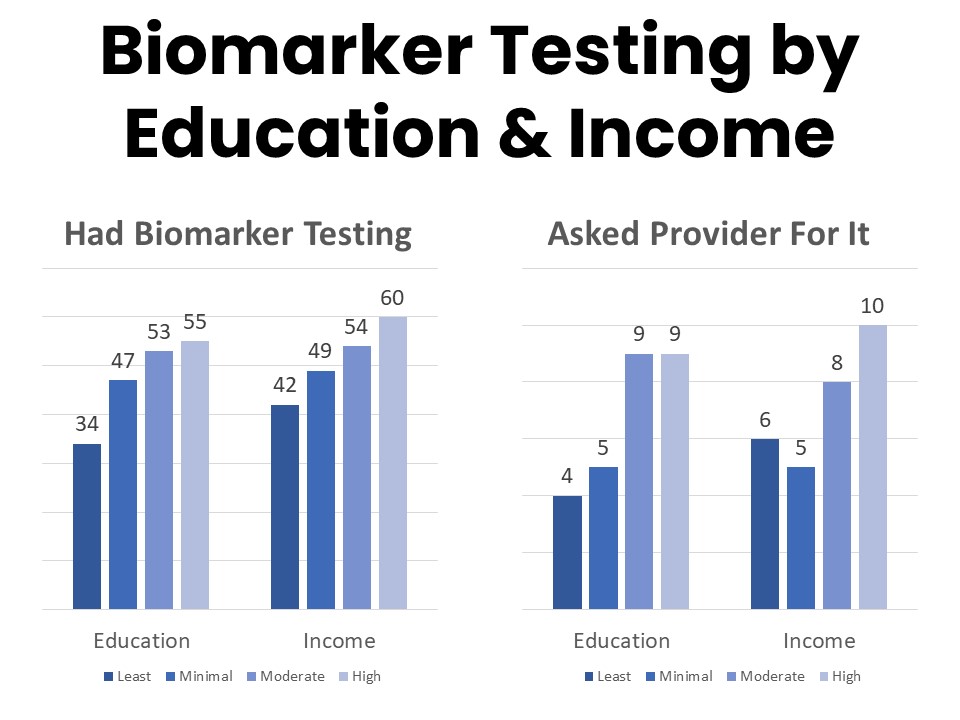
Biomarker testing was covered by insurance for 76% of those who had the test and half had no out of pocket costs. Those enrolled in Medicare, Medicaid, or an employer plan are more likely to report coverage for biomarker testing than those using a plan purchased privately such as through the marketplace. Nine percent of those who did not have biomarker testing say it was because of cost or coverage concerns. This is a significant improvement since the last time this question was asked in 2020, when 29% mentioned cost and coverage as reasons they did not have biomarker testing.
Over three-quarters (77%) of those who have had biomarker testing agree that it gave their providers valuable information that improved their ability to treat the patient’s cancer. Fifty-three percent say they are more likely to recover because of the biomarker testing. Half say they were able to avoid unnecessary treatments or procedures because they had biomarker testing. The results of biomarker testing led to changes in treatment protocol for 23%, and allowed 3% to find and enroll in a clinical trial.
Many of those who did not have biomarker testing (35%) simply weren’t a good candidate for it, but 69% never discussed it with a provider. Seventy-seven percent of those who did not have biomarker testing agree they would want it done if they were a good candidate for it.
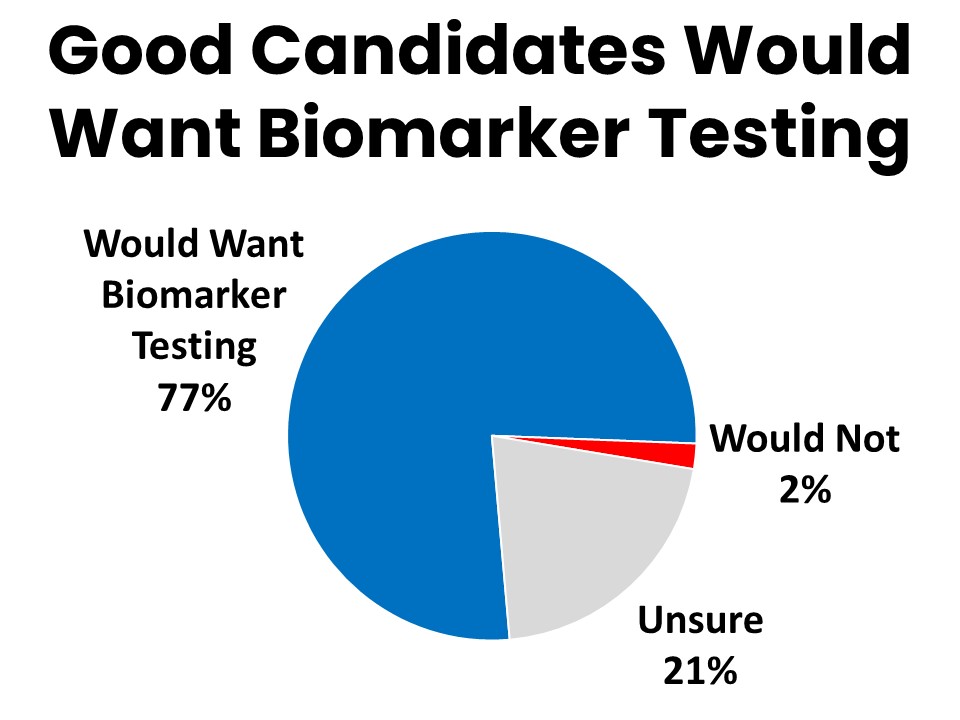
Cancer patients and survivors who have had biomarker testing describe many specific ways their experience benefitted from the testing, particularly when it comes to having the peace of mind that the best treatment plan was followed as a result. Some representative quotes from patients and survivors follow:
How might your experience have been different if you hadn’t had biomarker testing?
“I was given 6 months to a year to live. The biomarkers led me to an immunotherapy trial that saved my life.”
“Biomarker testing entered me into a treatment plan that has given me a complete response after failure of two prior plans.”
“We would be less certain about the optimal treatment plan, based on clinical knowledge and standards in place today.”
Maintaining Access to Telehealth Services from Out of State Providers is Important to Cancer Patients and Survivors
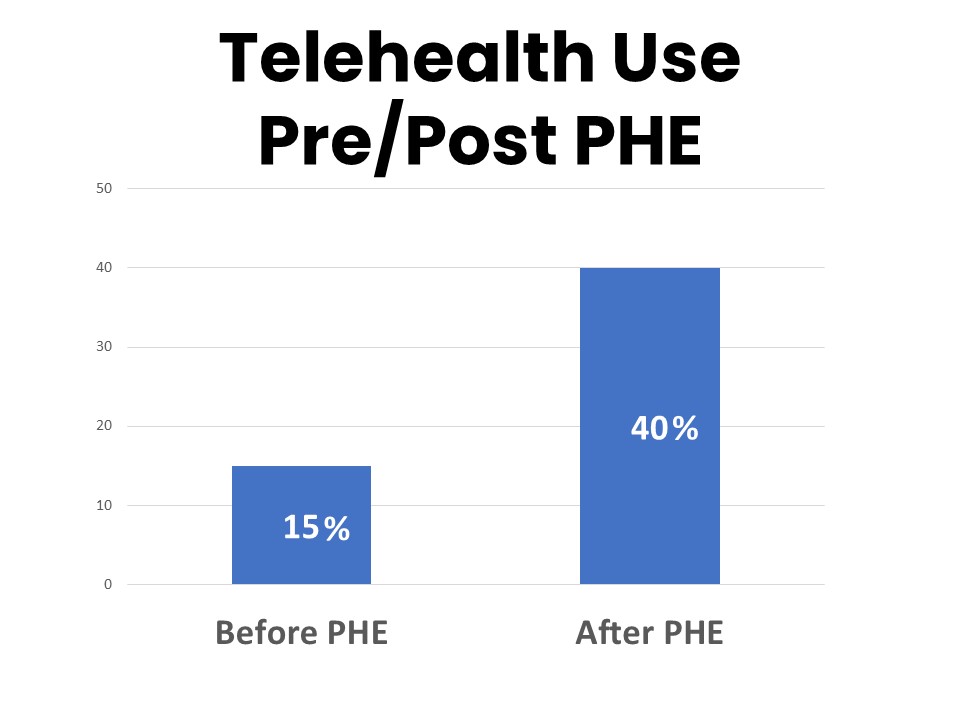
Over one-third of cancer patients and survivors have had a telehealth visit (video or phone call) with a provider regarding an issue related to their cancer that they would otherwise have addressed in an office visit. While most of those telehealth visits (78%) took place during the Covid-19 Public Health Emergency (PHE), the number reporting telehealth visits conducted outside of the PHE climbed from 15% prior to the PHE to 40% who have continued to have telehealth visits since the end of the PHE in May 2023. Forty-two percent say they plan to continue using telehealth visits with health care providers about issues related to their cancer.
Fifteen percent of those surveyed have seen a provider located in a different state from the state they live in. These visits are most often with specific providers such as specialists (74%), but also encompass imaging (57%), surgical (54%), lab testing (53%), regular oncology provider visits (32%), infusions (29%), second opinions (24%) and radiation (21%) appointments. Among those who have had telehealth visits, 16% were with providers located out of state. Fifty-nine percent say it is important that they personally are able to have telehealth visits with providers for their cancer care even if the provider is located out of state, and 79% agree it is important for cancer patients and survivors in general to have access to telehealth services from health care providers in other states.
Methodology
ACS CAN’s Survivor Views research initiative was designed to support the organization’s efforts to end suffering and death from cancer through public policy advocacy. Data provided by cancer patients and survivors as part of this project allows for a greater understanding of their experiences and opinions on cancer-related issues and gives voice to cancer patients and survivors in the shaping and advocating of public policies that help prevent, detect, and treat cancer and promote a more positive quality of life for those impacted.
To ensure the protection of all participants in this initiative all research protocols, questionnaires, and communications are reviewed by the Morehouse School of Medicine Institutional Review Board.
The survey population is comprised of individuals who meet the following criteria:
- Diagnosed with and/or treated for cancer within the last seven years
- Over the age of 18 (parents of childhood cancer survivors were invited to participate on behalf of their minor children)
- Reside in the US or US territories
Potential Survivor Views participants were invited to participate through email invitations, social media promotion, and partner group outreach. Those who agreed to participate after reviewing the informed consent information completed a brief survey including demographic and cancer history information to inform analysis as well as topical questions as discussed in this document. The data were collected between September 12-25, 2023. A total of 1,222 cohort participants responded to the survey. Differences reported between groups are tested for statistical significance at a 95% confidence interval.
About ACS CAN
The American Cancer Society Cancer Action Network (ACS CAN) advocates for evidence-based public policies to reduce the cancer burden for everyone. We engage our volunteers across the country to make their voices heard by policymakers at every level of government. We believe everyone should have a fair and just opportunity to prevent, detect, treat, and survive cancer. Since 2001, as the American Cancer Society’s nonprofit, nonpartisan advocacy affiliate, ACS CAN has successfully advocated for billions of dollars in cancer research funding, expanded access to quality affordable health care, and advanced proven tobacco control measures. We stand with our volunteers, working to make cancer a top priority for policymakers in cities, states and our nation’s capital. Join the fight by visiting www.fightcancer.org.