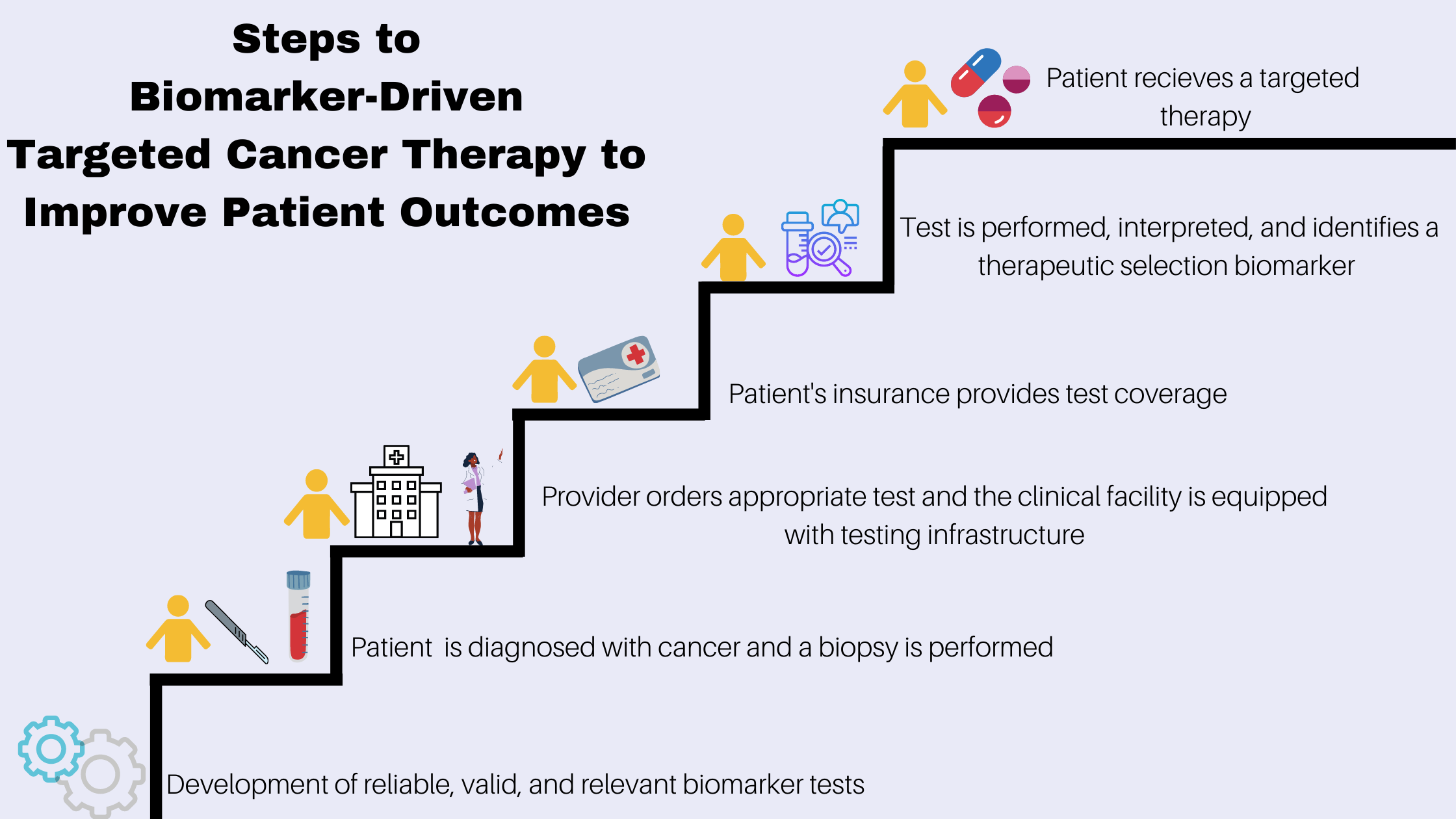
 The knowledge and practice of precision medicine in cancer have been progressing rapidly and advances have led to targeted cancer therapies that can improve patient survival and quality life. Treatment with targeted therapy often requires diagnostic testing to analyze biological samples taken from patients to identify and evaluate cancer biomarkers.
The knowledge and practice of precision medicine in cancer have been progressing rapidly and advances have led to targeted cancer therapies that can improve patient survival and quality life. Treatment with targeted therapy often requires diagnostic testing to analyze biological samples taken from patients to identify and evaluate cancer biomarkers.
Testing patients for specific biomarkers is integral to precision medicine in cancer care, but despite evidence pointing to the clinical benefits associated with biomarker testing, routine clinical use does not always follow, and testing rates lag behind clinical guideline recommendations. As highlighted in a recent ACS CAN survey of cancer patients and survivors, only one-third of respondents reported having biomarker testing. Of those who reported not receiving biomarker testing, more than a quarter said it was because insurance would not cover it or their out-of-pocket costs would be too high.
A new report by ACS CAN and LUNGevity Foundation found while health insurance coverage for biomarker testing has improved since the organizations’ last report in 2018, coverage is still failing to keep up with innovation and large coverage gaps remain. Patient access to appropriate biomarker testing relies on a combination of factors, as highlighted in the ACS CAN report Improving Access to Biomarker Testing - Advancing Precision Medicine in Cancer Care which explores the current landscape of cancer biomarker testing, sheds light on the nature of challenges limiting adoption of appropriate testing, and proposes recommendations to increase the uptake of testing and advance the use of precision medicine in cancer.
 The knowledge and practice of precision medicine in cancer have been progressing rapidly and advances have led to targeted cancer therapies that can improve patient survival and quality life. Treatment with targeted therapy often requires diagnostic testing to analyze biological samples taken from patients to identify and evaluate cancer biomarkers.
The knowledge and practice of precision medicine in cancer have been progressing rapidly and advances have led to targeted cancer therapies that can improve patient survival and quality life. Treatment with targeted therapy often requires diagnostic testing to analyze biological samples taken from patients to identify and evaluate cancer biomarkers.